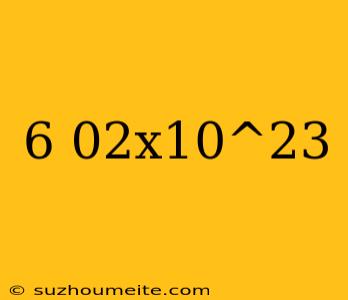The Magic Number: 6.02 x 10^23
In the world of chemistry, there exists a number that is so fundamental, so crucial, and so ubiquitous that it has become a benchmark for measuring the quantity of particles in a given substance. This number is none other than 6.02 x 10^23, also known as Avogadro's number.
Who is Avogadro?
Amedeo Avogadro was an Italian chemist who lived in the 18th and 19th centuries. He is credited with formulating the hypothesis that equal volumes of gases at the same temperature and pressure contain an equal number of molecules. This hypothesis, which is now known as Avogadro's law, was a groundbreaking concept in the field of chemistry.
What is Avogadro's Number?
Avogadro's number is a constant that represents the number of particles (atoms or molecules) in one mole of a substance. It is defined as 6.02 x 10^23 particles, where the ^ symbol indicates exponentiation. In simpler terms, it is the number of atoms or molecules in a single mole of a substance.
Importance of Avogadro's Number
Avogadro's number has far-reaching implications in the field of chemistry. It provides a standardized unit of measurement for the amount of a substance, allowing chemists to calculate the number of particles in a given sample with ease. This, in turn, enables chemists to:
- Calculate the molecular weight of a substance
- Determine the amount of a substance required for a reaction
- Analyze the composition of a mixture
- And many more...
Real-World Applications
Avogadro's number has numerous applications in various fields, including:
- Pharmaceuticals: Accurate dosing of medication relies on the precise measurement of particles, which is made possible by Avogadro's number.
- Materials Science: The number of particles in a material affects its properties, such as strength, conductivity, and optical properties.
- Food Industry: Food manufacturers use Avogadro's number to ensure the correct dosage of ingredients and additives.
Conclusion
In conclusion, Avogadro's number is a fundamental constant that has revolutionized the field of chemistry. Its importance cannot be overstated, as it provides a standardized unit of measurement for the amount of a substance. Without this number, many chemical reactions and processes would be impossible to quantify and control.