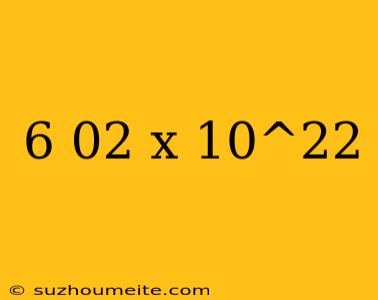The Avogadro Constant: 6.02 x 10^22
What is the Avogadro Constant?
The Avogadro constant is a fundamental constant in chemistry and physics that represents the number of particles (atoms or molecules) in one mole of a substance. It is named after the Italian scientist Amedeo Avogadro, who first proposed the concept of the mole as a unit of measurement in the early 19th century.
The Value of the Avogadro Constant
The Avogadro constant is defined as 6.02 x 10^22 particles per mole. This number is a fundamental constant of nature, and it is used to convert between the amount of a substance and its physical properties, such as volume and mass.
Importance of the Avogadro Constant
The Avogadro constant is a crucial constant in many areas of science, including chemistry, physics, and biology. It is used to:
- Define the mole: The Avogadro constant is used to define the mole, which is the unit of measurement for the amount of a substance.
- Convert between units: The Avogadro constant is used to convert between different units of measurement, such as from moles to grams or from liters to cubic centimeters.
- Calculate physical properties: The Avogadro constant is used to calculate physical properties, such as the volume and mass of a substance.
- Understand chemical reactions: The Avogadro constant is used to understand chemical reactions, including the stoichiometry of reactions and the calculations of reaction rates.
Applications of the Avogadro Constant
The Avogadro constant has many practical applications in various fields, including:
- Chemical engineering: The Avogadro constant is used to design and optimize chemical plants and processes.
- Materials science: The Avogadro constant is used to study the properties of materials and their applications.
- Pharmaceuticals: The Avogadro constant is used to develop and manufacture drugs and medicines.
- Environmental science: The Avogadro constant is used to study and mitigate the effects of pollution and environmental degradation.
Conclusion
In conclusion, the Avogadro constant is a fundamental constant in science that represents the number of particles in one mole of a substance. It is a crucial constant that is used in many areas of science and has many practical applications in various fields.