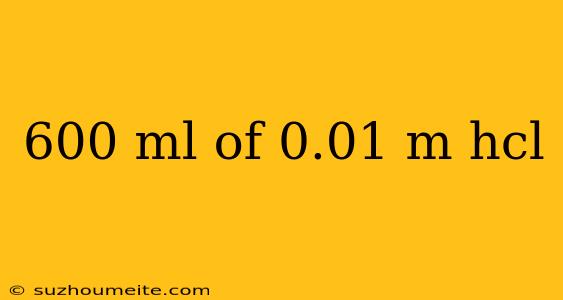600 ml of 0.01 M HCl
Introduction
In this article, we will discuss the characteristics and properties of 600 ml of 0.01 M HCl solution. Hydrochloric acid (HCl) is a strong acid commonly used in various industrial and laboratory applications.
Molarity
The molarity of a solution is defined as the number of moles of solute per liter of solution. In this case, the molarity of the HCl solution is 0.01 M, which means that there are 0.01 moles of HCl per liter of solution.
Volume
The volume of the solution is 600 ml, which is equivalent to 0.6 liters. Therefore, the number of moles of HCl in the solution can be calculated as follows:
Number of moles = Molarity x Volume (in liters) = 0.01 M x 0.6 L = 0.006 moles
Concentration
The concentration of the HCl solution is 0.01 M, which means that the solution contains 0.01 moles of HCl per liter of solution. This is a relatively dilute solution, compared to concentrated HCl solutions which can have molarities of up to 12 M.
Properties
Physical Properties
- Color: Colorless
- Odor: Pungent, acidic
- Density: approximately 1.0 g/ml
- Boiling Point: approximately 110°C
- Melting Point: approximately -30°C
Chemical Properties
- pH: The pH of the solution will be acidic, with a value of around 2-3.
- Reactivity: HCl is a strong acid and will react with bases to form salts and water.
Uses
- Cleaning and etching of surfaces
- Production of pharmaceuticals and dyes
- Food processing and preservation
- Production of steel and other metals
Safety Precautions
- Handle with care, as HCl is corrosive and can cause skin and eye irritation.
- Wear protective gear, including gloves and goggles, when handling the solution.
- Avoid inhalation of the vapors, as they can cause respiratory problems.
In conclusion, 600 ml of 0.01 M HCl solution is a relatively dilute solution of hydrochloric acid, with a concentration of 0.01 moles per liter. It has a range of physical and chemical properties, and is used in various industrial and laboratory applications. However, it requires careful handling and safety precautions to avoid accidents and injuries.