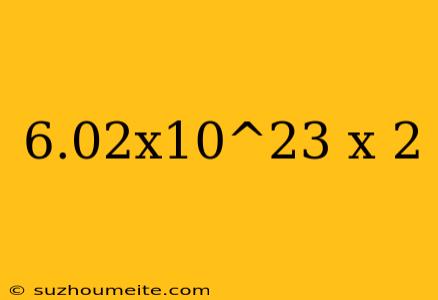The Power of Avogadro's Number: 6.02x10^23 x 2
In the world of chemistry and physics, there exists a fundamental constant that plays a crucial role in our understanding of the molecular structure of substances. This constant is known as Avogadro's number, denoted by the symbol NA. In this article, we will explore the significance of Avogadro's number and calculate the result of multiplying it by 2.
What is Avogadro's Number?
Avogadro's number is a dimensionless quantity that represents the number of particles (atoms or molecules) in one mole of a substance. It is named after the Italian scientist Amedeo Avogadro, who first proposed the concept in 1811. The currently accepted value of Avogadro's number is:
NA = 6.022140857 x 10^23 particles per mole
Calculating 6.02x10^23 x 2
Now, let's perform the calculation:
6.02 x 10^23 x 2 = ?
To evaluate this expression, we need to follow the order of operations (PEMDAS):
- Multiply 6.02 x 10^23 by 2:
6.02 x 10^23 x 2 = 12.04 x 10^23
Therefore, the result of multiplying Avogadro's number by 2 is approximately 12.04 x 10^23 particles.
Significance of Avogadro's Number
Avogadro's number has far-reaching implications in various fields, including:
- Stoichiometry: Avogadro's number is used to calculate the amount of substances required for a chemical reaction.
- Molar mass: The number is used to calculate the molar mass of a substance, which is essential in chemical reactions.
- Gas laws: Avogadro's number is used to derive the ideal gas law, which describes the behavior of ideal gases.
In conclusion, Avogadro's number is a fundamental constant that plays a vital role in our understanding of the molecular structure of substances. Multiplying it by 2 results in a staggering 12.04 x 10^23 particles, highlighting the immense scale of chemical reactions and the importance of Avogadro's number in chemistry and physics.