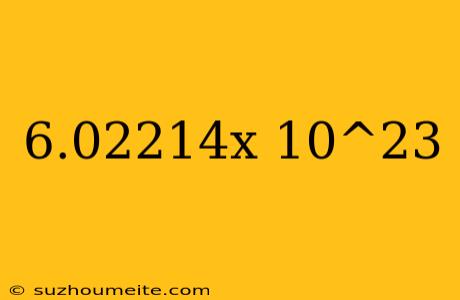The Magic Number: 6.02214 x 10^23
Avogadro's Constant: A Fundamental Constant in Chemistry
In the vast world of chemistry, there exist certain numbers that hold immense significance. One such number is 6.02214 x 10^23, also known as Avogadro's constant. This constant is a fundamental concept in chemistry, and its importance cannot be overstated.
What is Avogadro's Constant?
Avogadro's constant is a dimensionless quantity that represents the number of particles (atoms or molecules) in one mole of a substance. A mole is a unit of measurement in chemistry that is equivalent to 6.02214 x 10^23 particles. This constant was first introduced by Italian scientist Amedeo Avogadro in the early 19th century.
The Importance of Avogadro's Constant
Avogadro's constant is crucial in chemistry because it provides a way to express the amount of a substance in a reproducible and consistent manner. This constant is used in various chemical calculations, such as determining the molar mass of a substance, calculating the number of moles of a reactant or product, and identifying the stoichiometry of a chemical reaction.
Real-World Applications
Avogadro's constant has numerous real-world applications in various fields, including:
Chemical Synthesis
In chemical synthesis, Avogadro's constant is used to calculate the amount of reactants required to produce a specific amount of product.
Pharmaceutical Industry
In the pharmaceutical industry, Avogadro's constant is used to determine the exact amount of active ingredients in a drug, ensuring consistency and accuracy in medication production.
Environmental Monitoring
Avogadro's constant is used in environmental monitoring to calculate the amount of pollutants in the air, water, and soil, enabling scientists to track and mitigate environmental pollution.
Conclusion
In conclusion, 6.02214 x 10^23, Avogadro's constant, is a fundamental constant in chemistry that has far-reaching implications in various fields. Its importance lies in its ability to provide a consistent and reproducible way of expressing the amount of a substance, enabling scientists to make accurate calculations and measurements.