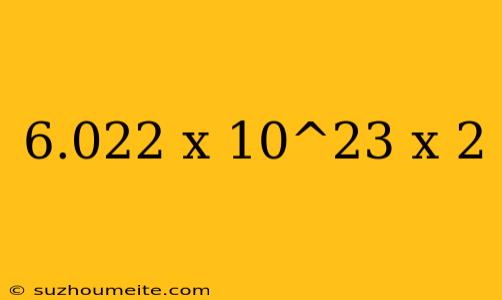The Mysterious Calculation: 6.022 x 10^23 x 2
Have you ever stumbled upon a complex calculation that left you wondering what it could possibly represent? Today, we're going to unravel the mystery behind the calculation 6.022 x 10^23 x 2. Buckle up, because we're about to dive into the world of chemistry and physics!
The Avogadro Constant
The calculation begins with the Avogadro constant, denoted by NA. This fundamental constant in chemistry and physics is named after the Italian scientist Amedeo Avogadro, who first proposed it in 1811. The Avogadro constant represents the number of particles (atoms or molecules) in one mole of a substance. It is defined as:
NA = 6.022 x 10^23 particles per mole
The Power of 10^23
The exponent 23 in the Avogadro constant is a staggering number. To put it into perspective, 10^23 is a 1 followed by 23 zeros! This enormous value represents the number of particles in a mole of a substance, which is a unit of measurement in chemistry.
Doubling the Fun: x 2
Now, let's get to the second part of the calculation: multiplying the Avogadro constant by 2. This could represent various things, depending on the context. Here are a few possibilities:
- Molecules vs. Atoms: If we're dealing with molecules, multiplying the Avogadro constant by 2 could represent the number of atoms in a mole of a molecule. For example, if we have a mole of oxygen molecules (O2), we would have 2 x NA atoms of oxygen.
- Dipoles and Pairs: In physics, the calculation could represent the number of dipoles or pairs of particles in a system. For instance, in a magnetic material, each atom could have a magnetic dipole, and multiplying the Avogadro constant by 2 would give us the total number of dipoles in a mole of the material.
Conclusion
The calculation 6.022 x 10^23 x 2 might seem daunting at first, but by breaking it down into its components, we can gain a deeper understanding of the underlying concepts. Whether it's molecules, atoms, dipoles, or pairs, this calculation is a testament to the intricate beauty of chemistry and physics. So, the next time you encounter a puzzling calculation, remember to take it apart and appreciate the fascinating world of science that lies beneath!