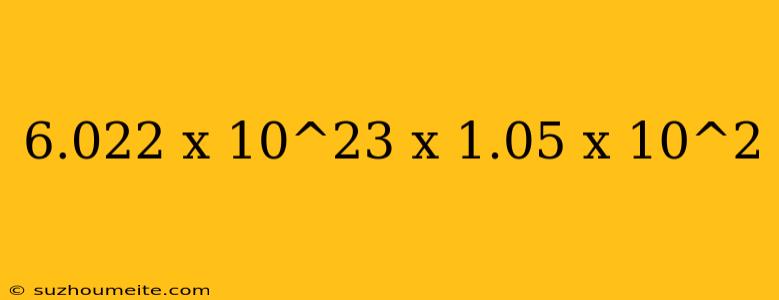The Mysterious World of Avogadro's Number
In the realm of chemistry, there exists a fundamental constant that plays a crucial role in understanding the behavior of molecules and atoms. This constant is known as Avogadro's number, denoted by the symbol NA, and is equal to 6.022 x 10^23.
What is Avogadro's Number?
Avogadro's number is a dimensionless quantity that represents the number of particles (atoms or molecules) in one mole of a substance. The concept of Avogadro's number was first introduced by the Italian scientist Amedeo Avogadro in 1811. It is a fundamental constant in chemistry and physics, used to express the amount of a substance in a given sample.
The Calculation: 6.022 x 10^23 x 1.05 x 10^2
Let's break down the calculation:
- 6.022 x 10^23: This is the value of Avogadro's number, which represents the number of particles in one mole of a substance.
- 1.05 x 10^2: This is a conversion factor, which is equivalent to 105.
When we multiply Avogadro's number by the conversion factor, we get:
6.022 x 10^23 x 1.05 x 10^2 = 6.3131 x 10^25
This calculation yields a massive number, representing the total number of particles in a given sample.
Importance of Avogadro's Number
Avogadro's number has far-reaching implications in various fields, including:
- Chemical reactions: Avogadro's number helps calculate the number of reactants and products involved in a chemical reaction.
- Stoichiometry: It aids in determining the quantitative relationships between reactants and products.
- Physical chemistry: Avogadro's number is used to calculate the properties of gases, such as volume, pressure, and temperature.
In conclusion, Avogadro's number is a fundamental constant that plays a vital role in understanding the behavior of molecules and atoms. The calculation 6.022 x 10^23 x 1.05 x 10^2 is a testament to the importance of this constant in chemistry and physics.