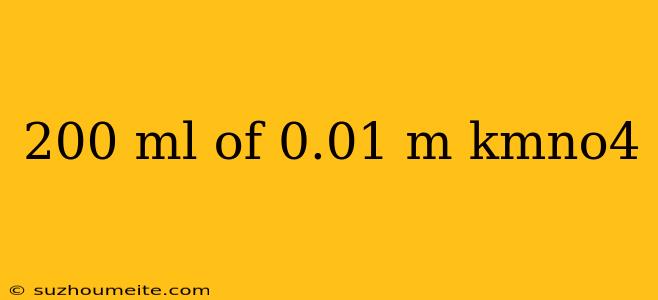200 mL of 0.01 M KMnO4: Understanding the Molar Concentration
In chemistry, concentrations of solutions are often expressed in terms of molar concentration, which is the number of moles of solute per liter of solution. In this article, we will explore the concept of molar concentration using the example of 200 mL of 0.01 M KMnO4 solution.
What is KMnO4?
KMnO4, also known as potassium permanganate, is a strong oxidizing agent commonly used in various chemical reactions. It is a purple crystalline solid that is highly soluble in water.
What does 0.01 M mean?
The "M" in 0.01 M KMnO4 stands for molar concentration, which is the number of moles of solute per liter of solution. In this case, 0.01 M means that there is 0.01 mole of KMnO4 per liter of solution.
How many moles of KMnO4 are in 200 mL?
To find the number of moles of KMnO4 in 200 mL of 0.01 M solution, we need to convert the volume from milliliters (mL) to liters (L) first.
1 L = 1000 mL 200 mL = 0.2 L
Now, we can use the molar concentration to find the number of moles:
Moles = Molar concentration x Volume (in liters) = 0.01 M x 0.2 L = 0.002 moles
How many grams of KMnO4 are in 200 mL?
To find the mass of KMnO4 in 200 mL of 0.01 M solution, we need to know the molar mass of KMnO4.
Molar mass of KMnO4 = 158.03 g/mol
Now, we can use the number of moles to find the mass:
Mass = Number of moles x Molar mass = 0.002 moles x 158.03 g/mol = 0.316 g
Conclusion
In conclusion, 200 mL of 0.01 M KMnO4 solution contains 0.002 moles of KMnO4, which is equivalent to 0.316 grams. Understanding molar concentration is essential in chemistry, as it allows us to accurately calculate the amounts of substances involved in chemical reactions.