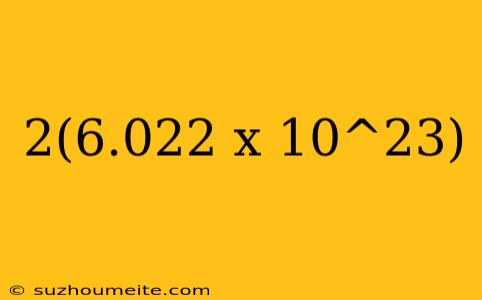The Power of Avogadro's Number: Unpacking 2(6.022 x 10^23)
Introduction
In the world of chemistry and physics, there exists a fundamental constant that plays a crucial role in understanding the behavior of molecules and atoms. This constant is known as Avogadro's number, and it's denoted by the symbol NA. But what happens when we multiply Avogadro's number by 2, and how does it impact our understanding of the microscopic world?
Avogadro's Number: A Brief Background
Avogadro's number, named after the Italian scientist Amedeo Avogadro, represents the number of particles (atoms or molecules) in one mole of a substance. It's a fundamental constant in chemistry and physics, with a value of 6.022 x 10^23 particles per mole. This number is a crucial concept in understanding the behavior of gases, reactions, and the structure of matter.
2(6.022 x 10^23): What Does it Represent?
When we multiply Avogadro's number by 2, we get 2(6.022 x 10^23). This expression represents twice the number of particles in one mole of a substance. To put it into perspective, if we consider a mole of oxygen gas (O2), which contains 6.022 x 10^23 molecules, multiplying it by 2 would result in 12.044 x 10^23 molecules.
What are the Implications of 2(6.022 x 10^23)?
The implications of 2(6.022 x 10^23) are far-reaching and significant in various fields:
Chemical Reactions
In chemical reactions, the number of particles involved can significantly affect the outcome. Doubling the number of particles can alter the rate of reaction, the yield of products, and even the reaction pathway. Understanding the implications of 2(6.022 x 10^23) is crucial in designing and optimizing chemical reactions.
Thermodynamics
Thermodynamic properties, such as entropy and energy, are directly related to the number of particles in a system. By doubling the number of particles, we can expect significant changes in these properties, which is critical in understanding the behavior of gases and materials.
Materials Science
In materials science, the number of particles in a material can greatly influence its properties, such as strength, conductivity, and optical properties. 2(6.022 x 10^23) can represent a significant change in the material's behavior, making it essential to understand its implications in material design and engineering.
Conclusion
In conclusion, 2(6.022 x 10^23) is more than just a mathematical expression – it represents a fundamental shift in our understanding of the microscopic world. By grasping the implications of this expression, we can gain insights into the behavior of particles, chemical reactions, thermodynamics, and materials science. As we continue to explore the intricacies of the microscopic world, the power of Avogadro's number and its multiples will remain a crucial foundation in our pursuit of knowledge.