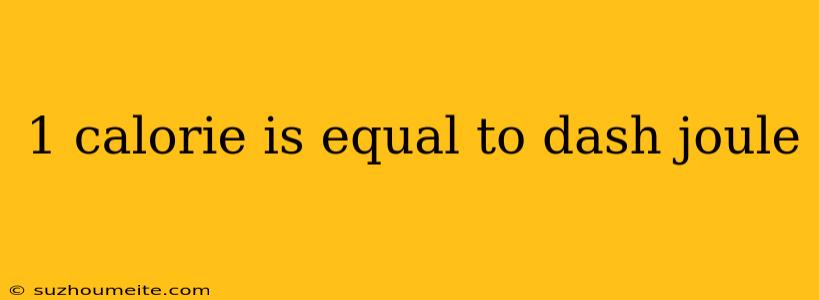1 Calorie is Equal to How Many Joules?
When it comes to measuring energy, two units come to mind: calories and joules. While both units are used to express energy, they are not interchangeable. In this article, we'll delve into the world of energy units and explore the relationship between calories and joules.
What is a Calorie?
A calorie is a unit of energy that is commonly used to express the energy content of foods and beverages. It is defined as the energy required to raise the temperature of one kilogram of water by one degree Celsius. In the context of nutrition, calories are used to measure the energy content of foods and beverages, and are an important factor in maintaining a healthy weight and overall health.
What is a Joule?
A joule is the International System of Units (SI) unit of energy. It is defined as the energy expended when a force of one newton is applied over a distance of one meter. Joules are used to measure energy in a wide range of fields, including physics, chemistry, and engineering.
The Conversion: 1 Calorie = How Many Joules?
So, how do calories and joules relate to each other? The answer lies in the conversion factor. One calorie is equal to approximately 4.184 joules. This means that if you consume a food item that contains 100 calories, it actually contains approximately 418.4 joules of energy.
Why is this Conversion Important?
Understanding the conversion between calories and joules is important for several reasons:
- Accurate energy measurement: By knowing the conversion factor, scientists and researchers can accurately measure energy outputs and inputs in various fields, from nutrition to physics.
- Practical applications: The conversion factor is essential in fields like engineering, where energy calculations are critical for designing and optimizing systems.
- Interdisciplinary collaboration: The conversion factor facilitates collaboration between researchers from different fields, ensuring that energy measurements are consistent and accurate.
In Conclusion
In conclusion, 1 calorie is equal to approximately 4.184 joules. This conversion factor is crucial in understanding energy measurements across various fields, from nutrition to physics. By recognizing the relationship between calories and joules, we can ensure accurate measurements and facilitate interdisciplinary collaboration.