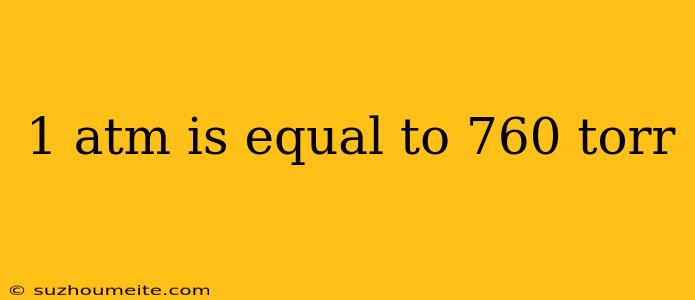1 ATM is Equal to 760 Torr: Understanding the Pressure Units
In the world of physics and chemistry, pressure is a fundamental concept that is used to describe the force exerted per unit area. There are several units of pressure, and two of the most commonly used units are atmospheres (atm) and torr. In this article, we will explore the relationship between these two units and why 1 atm is equal to 760 torr.
What is Atmospheric Pressure?
Atmospheric pressure, also known as atmospheric force, is the pressure exerted by the weight of the air in the atmosphere. It is the pressure that surrounds us every day, and it is measured in units of pressure such as atmospheres (atm) or pounds per square inch (psi). At sea level, the atmospheric pressure is approximately 1 atm, which is equivalent to 1013 millibars (mbar) or 760 torr.
What is a Torr?
A torr is a unit of pressure that is named after the Italian physicist Evangelista Torricelli. It is defined as the pressure required to support a column of mercury (Hg) 1 mm high. The torr is a smaller unit of pressure compared to the atmosphere, and it is commonly used in scientific research and applications where high-precision pressure measurements are required.
The Relationship Between 1 atm and 760 torr
The relationship between 1 atm and 760 torr is a simple one. One atmosphere (1 atm) is equal to 760 torr. This means that if a pressure is measured as 1 atm, it is equivalent to a pressure of 760 torr.
Why is 1 atm Equal to 760 torr?
The reason why 1 atm is equal to 760 torr is due to the definition of these units. The atmosphere is defined as the pressure required to support a column of mercury (Hg) 760 mm high, while the torr is defined as the pressure required to support a column of mercury (Hg) 1 mm high. Therefore, 1 atm is equivalent to 760 torr because it takes 760 times the pressure of 1 torr to equal the pressure of 1 atm.
Conclusion
In conclusion, 1 atm is equal to 760 torr due to the definition of these units. Understanding the relationship between these units is important in scientific research and applications where high-precision pressure measurements are required. By knowing that 1 atm is equal to 760 torr, scientists and engineers can accurately convert between these units and make precise measurements.