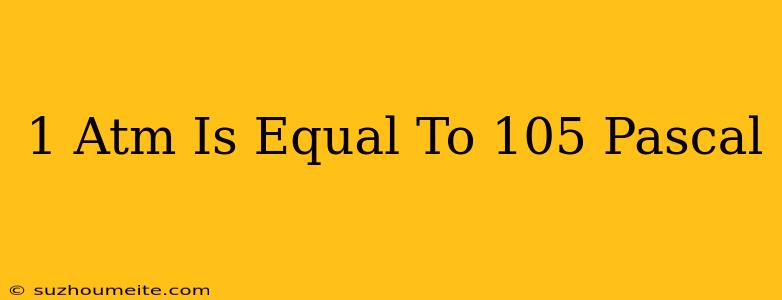1 ATM is Equal to 10^5 Pascal: Understanding Atmospheric Pressure
Introduction
Atmospheric pressure, also known as air pressure, is the pressure exerted by the weight of the air in the atmosphere. It is an important factor in many natural phenomena, including weather patterns, ocean currents, and even the behavior of living organisms. In this article, we will explore the relationship between ATM (atmosphere) and Pascal, and why 1 ATM is equal to 10^5 Pascal.
What is ATM?
ATM, or atmosphere, is a unit of pressure that is commonly used to express the pressure of gases, particularly in industrial and scientific applications. It is defined as the pressure exerted by a column of mercury 760 mm high at 0°C. This unit of pressure is widely used in many industries, including aerospace, chemical, and biomedical engineering.
What is Pascal?
Pascal, on the other hand, is the SI unit of pressure, named after the French mathematician and physicist Blaise Pascal. It is defined as one newton per square meter, or N/m². The Pascal is a smaller unit of pressure compared to the ATM, but it is more commonly used in scientific and engineering applications.
The Conversion: 1 ATM is Equal to 10^5 Pascal
Now, let's dive into the conversion. 1 ATM is equal to 10^5 Pascal, which means that one atmosphere of pressure is equivalent to 100,000 Pascals. This conversion is important in many applications, including aerospace engineering, where the pressure of the atmosphere is critical in designing aircraft and spacecraft.
To put this conversion into perspective, let's consider an example. The average atmospheric pressure at sea level is about 1 ATM, or 101,325 Pascals. This means that if you were to measure the pressure at sea level using a Pascal meter, you would get a reading of approximately 101,325 Pascals.
Conclusion
In conclusion, 1 ATM is equal to 10^5 Pascal, which is an important conversion to understand in many scientific and engineering applications. By grasping this conversion, we can better appreciate the complexity of atmospheric pressure and its importance in our daily lives. Whether you're an engineer designing aircraft or a scientist studying the atmosphere, understanding the relationship between ATM and Pascal is crucial for success.