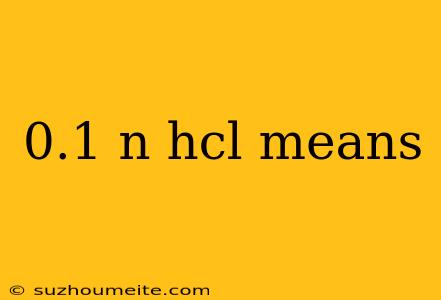0.1 N HCl: Understanding the Meaning and Significance
When working with chemicals, it's essential to understand the notation used to represent their concentration. One common notation is the normality (N) of a solution, which is often expressed in terms of a specific acid or base. In this article, we'll explore the meaning of 0.1 N HCl and its significance in various applications.
What is Normality (N)?
Normality (N) is a measure of the concentration of a solution, defined as the number of equivalent weights of a solute per liter of solution. In other words, it's a way to express the amount of a substance that can react with a certain amount of another substance. The equivalent weight is the mass of a substance that reacts with one equivalent of another substance.
What is 0.1 N HCl?
0.1 N HCl is a solution of hydrochloric acid (HCl) with a normality of 0.1. This means that one liter of this solution contains 0.1 equivalent weights of HCl. The equivalent weight of HCl is 36.46 grams per equivalent, so a 0.1 N HCl solution would contain 3.646 grams of HCl per liter.
Preparation of 0.1 N HCl
To prepare 0.1 N HCl, you can start with a concentrated HCl solution (typically 37% or 12 M) and dilute it with distilled water. The exact procedure may vary depending on the specific requirements and equipment available. Here's a general outline:
- Start with a certain volume of concentrated HCl solution.
- Calculate the amount of distilled water needed to achieve the desired normality.
- Slowly add the water to the HCl solution while stirring.
- Mix well and store the solution in a clean, labeled container.
Applications of 0.1 N HCl
0.1 N HCl has various applications in different fields, including:
Laboratory Settings
- Chemical analysis: 0.1 N HCl is used as a standard solution for volumetric analysis, such as titration reactions.
- pH adjustment: HCl solutions can be used to adjust the pH of a solution or buffer.
Industrial Applications
- Food processing: HCl is used as a food additive, pH controller, and cleaning agent in the food industry.
- Pharmaceuticals: HCl is used as an active ingredient, pH adjuster, and cleaning agent in pharmaceutical manufacturing.
Medical Applications
- Medical treatments: HCl is used in some medical treatments, such as gastric acid simulation and wound cleaning.
- Laboratory testing: 0.1 N HCl is used as a control solution in some laboratory tests, such as gastric acid secretion assays.
Conclusion
In conclusion, 0.1 N HCl is a solution of hydrochloric acid with a specific normality, which is used in various applications, including laboratory settings, industrial processes, and medical treatments. Understanding the meaning and significance of 0.1 N HCl is essential for accurate and safe handling of this chemical solution.