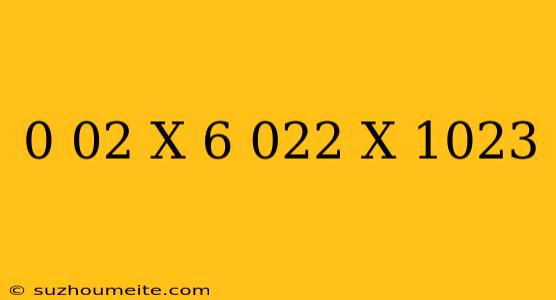The Amazing World of Avogadro's Number
In the realm of chemistry and physics, there exists a fundamental constant that plays a crucial role in understanding the behavior of matter at the atomic and molecular level. This constant is known as Avogadro's number, denoted by the symbol NA. In this article, we'll delve into the fascinating world of Avogadro's number and explore its significance in scientific calculations.
What is Avogadro's Number?
Avogadro's number is a dimensionless quantity that represents the number of particles (atoms or molecules) in one mole of a substance. It is named after the Italian scientist Amedeo Avogadro, who first proposed the idea in 1811. The currently accepted value of Avogadro's number is:
NA = 6.022 x 10^23 particles per mole
The Calculation: 0.02 x 6.022 x 10^23
Now, let's dive into the calculation that brought you to this article:
0.02 x 6.022 x 10^23 = ?
To evaluate this expression, we need to follow the order of operations (PEMDAS):
- Multiply 0.02 by 6.022: 0.02 x 6.022 = 0.12044
- Multiply the result by 10^23: 0.12044 x 10^23 = 1.2044 x 10^22
So, the result of the calculation is approximately:
1.2044 x 10^22 particles
What does this calculation mean?
In the context of chemistry, this calculation might represent the number of particles in a specific sample of a substance. For instance, if we have 0.02 moles of a compound, and we want to know the number of particles (atoms or molecules) present in that sample, we can use Avogadro's number to calculate the answer.
Conclusion
Avogadro's number is a fundamental constant that plays a vital role in scientific calculations, particularly in chemistry and physics. Understanding its significance and applications can help us better comprehend the behavior of matter at the atomic and molecular level. The calculation 0.02 x 6.022 x 10^23 might seem simple, but it highlights the importance of Avogadro's number in determining the number of particles in a given sample.