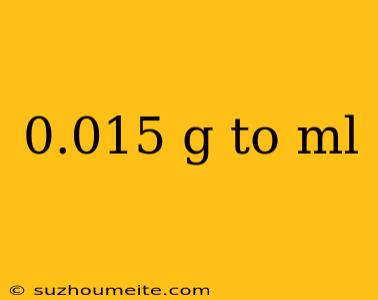Converting 0.015 g to mL: Understanding Density and Volume
When working with small quantities of substances, it's essential to understand the conversion between grams (g) and milliliters (mL). In this article, we'll explore how to convert 0.015 g to mL, and discuss the importance of density and volume in this process.
What is Density?
Density is a measure of mass per unit volume of a substance. It's typically expressed in units of grams per milliliter (g/mL) or kilograms per liter (kg/L). The density of a substance depends on its chemical composition and physical properties.
Converting 0.015 g to mL
To convert 0.015 g to mL, we need to know the density of the substance. Let's assume we're working with water, which has a density of approximately 1 g/mL.
** Formula: **
mL = g / density
Calculation:
mL = 0.015 g / 1 g/mL mL = 0.015 mL
Result:
0.015 g is equivalent to approximately 0.015 mL.
Important Considerations
When converting between grams and milliliters, it's crucial to consider the following factors:
- Density: Different substances have varying densities, which affect the conversion.
- Volume: The volume of a substance can change depending on temperature, pressure, and other conditions.
- Units: Ensure you're using the correct units for mass (g) and volume (mL).
Real-World Applications
Understanding the conversion between grams and milliliters is essential in various fields, including:
- Chemistry: Accurate measurements are critical in chemical reactions and experiments.
- Pharmaceuticals: Precise conversions are necessary when preparing medications and dosages.
- Cooking: Converting between grams and milliliters is useful when scaling recipes or measuring ingredients.
Conclusion
In conclusion, converting 0.015 g to mL requires knowledge of the substance's density and a basic understanding of the formula. By considering factors such as density, volume, and units, you can make accurate conversions and apply this knowledge in various real-world scenarios.