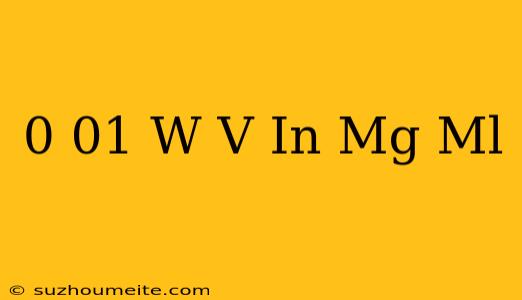0.01 w/v in mg/ml: Understanding Concentration Units
In chemistry and pharmacy, concentration units are used to express the amount of a substance dissolved in a solution. Two common concentration units are w/v (weight per volume) and mg/ml (milligrams per milliliter). In this article, we will discuss the conversion of 0.01 w/v to mg/ml and explore the significance of these units.
What does 0.01 w/v mean?
0.01 w/v means that 1 mL of the solution contains 0.01 grams or 10 milligrams of the substance. The "w/v" abbreviation stands for "weight per volume," indicating that the concentration is expressed as a ratio of the weight of the substance to the volume of the solution.
Converting 0.01 w/v to mg/ml
To convert 0.01 w/v to mg/ml, we need to recall that 1 mL is equal to 1 gram. Therefore, we can set up the following conversion:
0.01 g/mL = x mg/mL
Since 1 g is equal to 1000 mg, we can convert the weight units:
0.01 g/mL = 10 mg/mL
So, 0.01 w/v is equivalent to 10 mg/mL.
Importance of Concentration Units
Understanding concentration units is crucial in various fields, including pharmacy, chemistry, and biology. Accurate calculations of concentration are essential to ensure the efficacy and safety of medications, chemical reactions, and biological experiments.
Conclusion
In conclusion, 0.01 w/v is equivalent to 10 mg/mL. Understanding the conversion between these concentration units is vital in various scientific fields. By grasping the concepts of w/v and mg/ml, you can accurately calculate concentrations and ensure the success of your experiments and applications.